WebElements Periodic Table of the Elements | Lithium | radii
This WebElements periodic table page contains radii for the element lithium ... The term "atomic radius" is not particularly helpful although its use is widespread. ...
Atomic Size
Atomic radii and covalent radii, "Chemical Systems," Chemical Bond ... In a family--like from hydrogen to lithium to sodium on down--the atomic size increases. ...
The first property to explore is atomic radius.
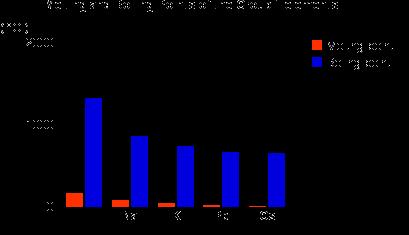
... notice from lithium to cesium, going down the group, the atomic radius increases. ... way to explain the trend in atomic radius across a period is in terms ...
Lithium
Features history, sources, its usage in WWII, and the price of the element. ... Atomic Radius: 152 pm. Atomic Symbol: Li. Melting Point: 180.5 ºC. Atomic Weight: 6.941 ...
atomic and ionic radius
Describes and explains how atomic radii vary around the Periodic Table ... From lithium to fluorine, those electrons are all in the 2-level, being screened ...
Periodic Table of Elements: Lithium ? Li ...
Comprehensive information for the element Lithium ? Li is provided by this page including scores of properties, element ... of Lithium. Atomic Radius: 2.05Å ...
Lithium - New World Encyclopedia
Atomic radius (calc.) 167 pm. Covalent radius. 134 pm. Van der Waals radius. 182 pm. Miscellaneous ... Lithium (chemical symbol Li, atomic number 3) is the ...
Periodic Trends in Atomic Properties
Properties such as the size of an atom (atomic radius), the energy required to ... 8. Given that the atomic radius and ionization energy of lithium are 152 pm and 520 ...
Lithium - Wikipedia, the free encyclopedia
... due to its small atomic radius or ionic radius. ... Lithium has a diagonal relationship with magnesium, an element of similar atomic and ionic radius. ...
Atomic and physical properties of Periodic Table Group 1
Explains the trends in atomic radius, first ionisation energy, electronegativity, melting point, boiling point and density for the Group 1 elements in the Periodic Table.